Molten Aluminium Refine Flux is mainly used to remove hydrogen and floating oxidized slag inside the aluminum liquid, so that the aluminum liquid is more pure, and it also has the effect of a slag cleaning agent.
Part of the components in the refining agent are easily decomposed at high temperatures, and the generated gas is prone to hydrogen reaction, has a strong adsorption force with slag inclusions, and quickly escapes from the melt. Other components also have the function of slag cleaning agent.
The flow degassing effect is mainly manifested in three aspects
1. Remove part of the composite hydrogen absorbed by oxidized inclusions.
2. When the flow interacts with the melt, gaseous products are formed to affect the diffusion and removal of hydrogen.
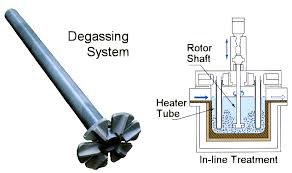
3. Since the oxide film on the surface of the melt is dissolved, the dissolved atomic hydrogen can easily diffuse into the atmosphere. However, the degassing effect of this flux is limited, and its hydrogen content can only be reduced to the level of 0.2-0.25mL/100gAl under production conditions. Most aluminum plants use online degassing devices for further purification.

The slag removal ability of Molten Aluminium Refine Flux depends on the flux’s adsorption and dissolution of oxidized inclusions in the melt and the chemical interaction between the flux and the melt.
Oxidized inclusions will not be wetted by molten aluminum, and the interfacial tension between the two is very important. The flux wets and oxidizes the inclusions, and the interfacial tension between the two is relatively low.
Once the flux absorbs the oxidized inclusions in the melt, it can reduce the free surface energy of the system.
Therefore, the flux has the ability to automatically adsorb oxidized inclusions, that is, the refining performance of Refine Flux.
This adsorption is the main reason for slag removal.
Obviously, the lower the interfacial tension between the flux and the non-metallic inclusions, the greater the interfacial tension between the flux and the molten aluminum, and the greater the interfacial tension between the molten aluminum and the non-metallic inclusions, the better the adsorption effect. Flow, the greater the slag removal effect.
The dissolving effect of the solvent on the oxide is determined by the nature of the flow.
Generally, when the molecular structure of the flux is similar to the substructure or chemical properties of certain oxides, mutual dissolution occurs at a certain temperature.
For example, Al2O3 and Na3AlF6 with the same cation have a certain degree of mutual solubility. However, in the normal refining temperature range of aluminum castings, the amount of oxides dissolved in the flux is very small.
After the chemical reaction between the solvent and the melt, the product is insoluble in aluminum.
When bubbles precipitate at the metal-oxide interface, the separation of the oxide film and the metal is promoted, and the oxide film is transferred to the water stream.
At the same time, bubbles also have the function of flotation and slag removal.
In addition, the bubbles formed during the flow dissociation process can also remove some inclusions by flotation.